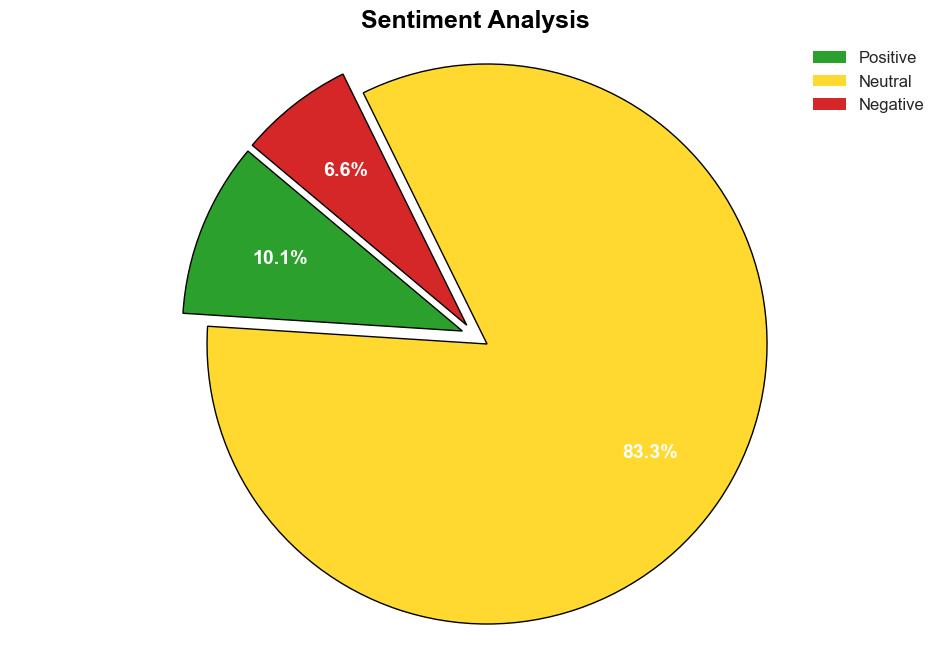
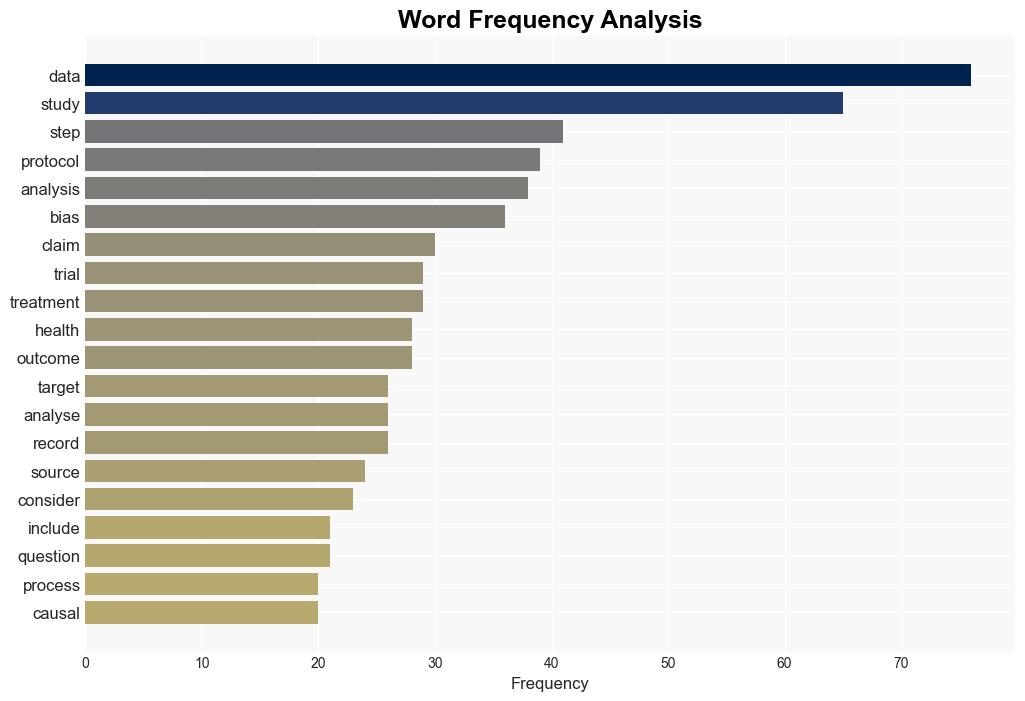
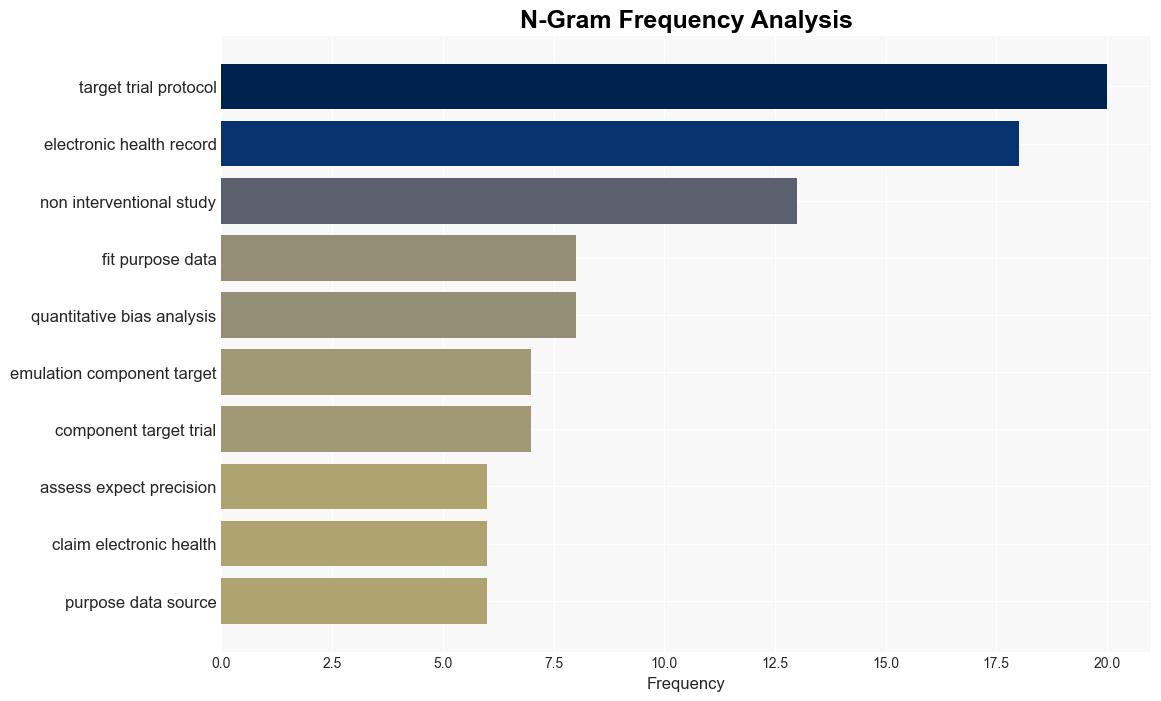
Process guide for inferential studies using healthcare data from routine clinical practice to evaluate causal effects of drugs PRINCIPLED considerations from the FDA Sentinel Innovation Center – The BMJ
Published on: 2024-02-12