Pharma’s Overlooked Cyber Threat: The Rise of Data Misuse and Compliance Risks
Published on: 2026-01-05
AI-powered OSINT brief from verified open sources. Automated NLP signal extraction with human verification. See our Methodology and Why WorldWideWatchers.
Intelligence Report: Pharmas most underestimated cyber risk isnt a breach
1. BLUF (Bottom Line Up Front)
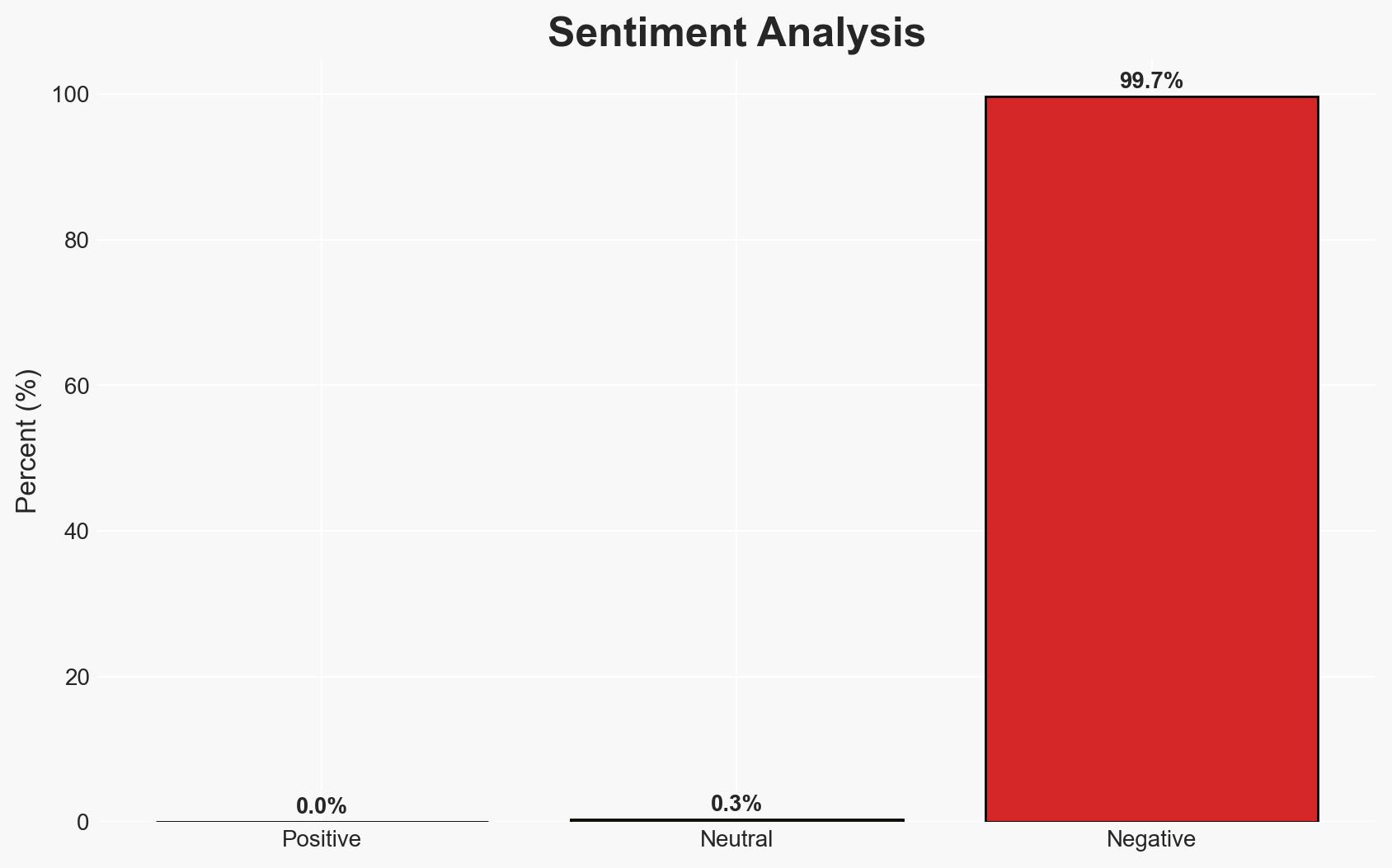
The primary cyber risk for pharmaceutical and life sciences companies is shifting from traditional breaches to data misuse and AI-driven exposure, exacerbated by inadequate real-time governance. This risk is underestimated due to a focus on breach prevention rather than silent control failures. The convergence of cybersecurity and compliance will necessitate new governance models. Overall confidence in this assessment is moderate.
2. Competing Hypotheses
- Hypothesis A: The primary cyber risk for pharma is still breaches and ransomware attacks. This is supported by the industry’s historical focus on breach prevention and the ongoing threat of ransomware. However, this view may overlook the evolving nature of data misuse risks.
- Hypothesis B: Data misuse and AI-driven exposure are the most significant emerging risks. This is supported by the increasing complexity of data flows and AI adoption, which create vulnerabilities not addressed by traditional compliance measures. Contradictory evidence includes the industry’s slow adaptation to these risks.
- Assessment: Hypothesis B is currently better supported due to the evolving nature of cyber threats and the inadequacy of existing compliance frameworks to address data misuse and AI-related risks. Indicators that could shift this judgment include significant breaches or ransomware incidents that refocus attention on traditional threats.
3. Key Assumptions and Red Flags
- Assumptions: Organizations will continue to prioritize breach prevention; AI adoption will increase without corresponding governance improvements; regulatory pressures will intensify.
- Information Gaps: Specific data on the frequency and impact of data misuse incidents; detailed understanding of AI systems’ data handling practices.
- Bias & Deception Risks: Potential bias in industry reporting towards traditional breach metrics; possible underreporting of data misuse incidents due to lack of detection.
4. Implications and Strategic Risks
The shift towards data misuse and AI-driven risks could lead to significant regulatory and operational challenges for the pharmaceutical industry. This evolution may strain existing compliance frameworks and necessitate new governance models.
- Political / Geopolitical: Increased regulatory scrutiny could lead to international tensions, especially if data misuse incidents involve cross-border data transfers.
- Security / Counter-Terrorism: The misuse of AI systems could create vulnerabilities exploitable by malicious actors, potentially impacting national security.
- Cyber / Information Space: The convergence of cybersecurity and compliance will require enhanced real-time governance and monitoring capabilities.
- Economic / Social: Data misuse incidents could undermine public trust in pharmaceutical companies, affecting market stability and social cohesion.
5. Recommendations and Outlook
- Immediate Actions (0–30 days): Conduct a comprehensive review of data governance practices; enhance monitoring of AI systems and vendor data flows.
- Medium-Term Posture (1–12 months): Develop partnerships with regulatory bodies to align on new compliance requirements; invest in real-time governance technologies.
- Scenario Outlook:
- Best: Industry adapts quickly, integrating new governance models, minimizing data misuse risks.
- Worst: Significant data misuse incidents lead to severe regulatory penalties and loss of public trust.
- Most-Likely: Gradual adaptation with periodic incidents prompting incremental improvements.
6. Key Individuals and Entities
- Chirag Shah, Global Information Security Officer & DPO at Model N
- Not clearly identifiable from open sources in this snippet.
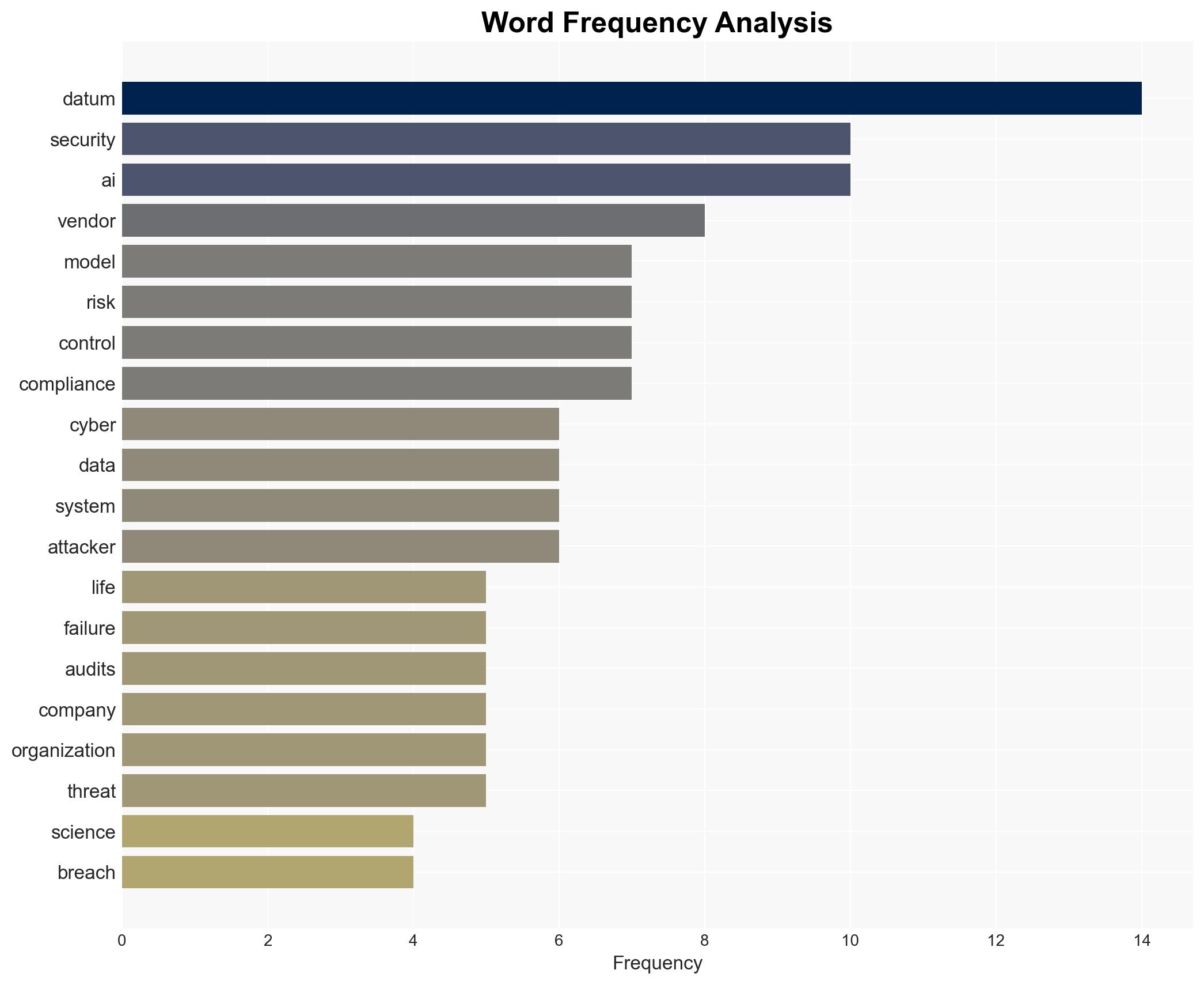
7. Thematic Tags
cybersecurity, data governance, AI exposure, pharmaceutical industry, compliance risk, data misuse, regulatory pressure
Structured Analytic Techniques Applied
- Adversarial Threat Simulation: Model hostile behavior to identify vulnerabilities.
- Indicators Development: Detect and monitor behavioral or technical anomalies across systems for early threat detection.
- Bayesian Scenario Modeling: Forecast futures under uncertainty via probabilistic logic.
Explore more:
Cybersecurity Briefs ·
Daily Summary ·
Support us